Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1


Chemistry, 22.06.2019 20:20, Matseleng3775
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 23:10, carmenguabaoql9kv
Afusion reaction takes place between carbon and another element. neutrons are released, and a different element is formed. the different element is a) lighter than helium. b)heavier than helium. c)the same weight as helium. d)dependent on the element that reacted with carbon.
Answers: 3
You know the right answer?
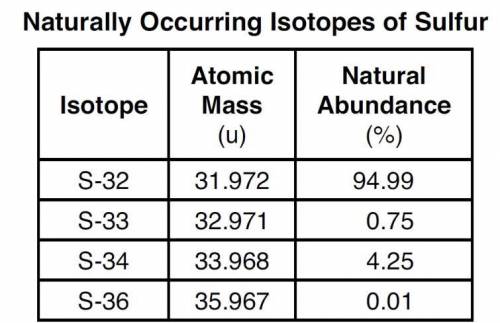
The four naturally occurring isotopes of sulfur are S-32, S-33, S-34, and S-36. The table below show...
Questions in other subjects:


Mathematics, 21.05.2020 22:59



Mathematics, 21.05.2020 22:59