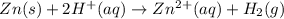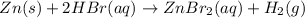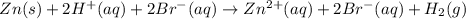Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 09:50, Amandachavez94
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1
You know the right answer?
The net ionic equation for the dissolution of zinc metal in aqueous hydrobromic acid is ....
Questions in other subjects:

Mathematics, 03.03.2021 17:30

Physics, 03.03.2021 17:30

Mathematics, 03.03.2021 17:30


Mathematics, 03.03.2021 17:30

Computers and Technology, 03.03.2021 17:30

Computers and Technology, 03.03.2021 17:30



Mathematics, 03.03.2021 17:30