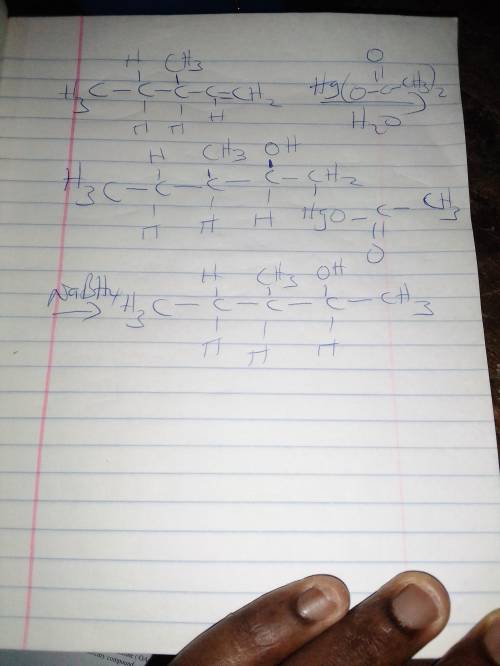Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:20, mathman783
Why does his teacher ask him to balance the equation by including the correct coefficient
Answers: 1

Chemistry, 22.06.2019 13:30, kassandrarosario1115
How many protons, electrons, and neutrons are in each of the following isotopes? a. zirconium-90 b. palladium-108 c. bromine-81 d. antimony-123
Answers: 1

Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1
You know the right answer?
When R-3-methylpent-1-ene is treated with aqueous mercury (II) acetate, then sodium borohydride, NaB...
Questions in other subjects:

World Languages, 24.09.2019 21:00




Social Studies, 24.09.2019 21:00





French, 24.09.2019 21:00