Chemistry, 20.12.2020 02:40 chumreaper2002
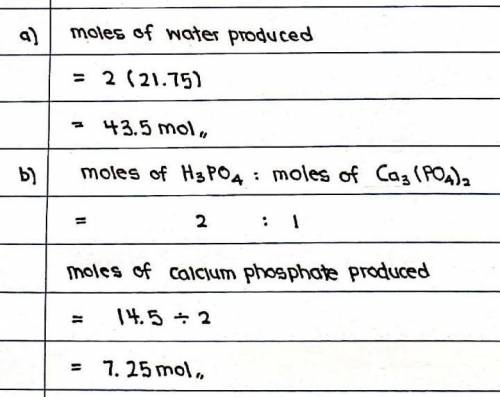
7. A. How many moles of Calcium hydroxide (CaOH2) are needed to completely react with 14.5 mol of Phosphoric acid (H3PO4)?
2 H3PO4 + 3 Ca(OH)2 → 6 H(OH) + Ca3(PO4)2
a. How many moles of water (HOH) will be produced?
b. How many moles of Calcium Phosphate (CaOH2) will be produced?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 22.06.2019 17:10, gungamer720
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
7. A. How many moles of Calcium hydroxide (CaOH2) are needed to completely react with 14.5 mol of Ph...
Questions in other subjects:


Mathematics, 10.02.2021 05:40

Mathematics, 10.02.2021 05:40


History, 10.02.2021 05:40

Mathematics, 10.02.2021 05:40



Mathematics, 10.02.2021 05:40