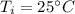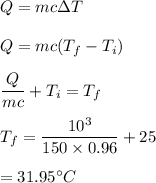Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 18:10, bri9263
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 19:30, dorindaramirez0531
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
8. If a sample of chloroform is initially at 25°C, what is its final temperature if 150.0 g of chlor...
Questions in other subjects:









Mathematics, 23.03.2020 22:38