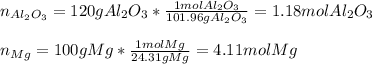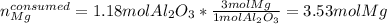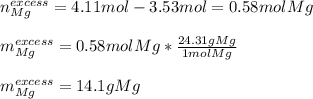Chemistry, 19.12.2020 06:30 aschunyhung
If 120 grams of aluminum oxide reacts with 100 grams of magnesium, which reactant is in excess and by how much? Al2O3 + 3Mg —> 3MgO + 2Al

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, asims13
Which element in the third period would you expect to have the larger atomic radius, sodium (na) or sulfur (s)? a. sodium, because it has a higher effective nuclear charge attracting electrons in fewer energy levels. b. sodium, because it has fewer protons attracting electrons in the same energy levels. c. sulfur, because it has more protons attracting electrons in more energy levels. d. sulfur, because it has a higher effective nuclear charge attracting electrons in the same energy levels.
Answers: 2


Chemistry, 22.06.2019 05:00, happy121906
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2
You know the right answer?
If 120 grams of aluminum oxide reacts with 100 grams of magnesium, which reactant is in excess and b...
Questions in other subjects:




Mathematics, 17.06.2021 02:00



Mathematics, 17.06.2021 02:00



Mathematics, 17.06.2021 02:00