
Chemistry, 19.12.2020 06:00 jsjsjsskakwkowwj
How would adding the catalyst nitrogen monoxide (NO) affect this reaction?
2SO2(g) + O2(g) → 2SO3(g)
A) NO increases the rate at which SO3 molecules are formed.
B) NO reacts with SO3 to produce more SO2 molecules.
C) NO decreases collisions between the SO2 and O2 molecules.
D) NO increases the concentration of the SO2 and O2 molecules.
E) NO increases the activation energy of the SO2 and O2 molecules.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2



Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
You know the right answer?
How would adding the catalyst nitrogen monoxide (NO) affect this reaction?
2SO2(g) + O2(g) → 2SO3(g...
Questions in other subjects:

Computers and Technology, 22.01.2021 17:30


Social Studies, 22.01.2021 17:30



English, 22.01.2021 17:30




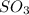 molecules are formed.
molecules are formed.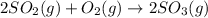
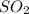 and
and 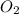 molecules will incraese which in turn will lead to formatioon of more
molecules will incraese which in turn will lead to formatioon of more 


