
Chemistry, 18.12.2020 21:00 kingcory717
Which of the following correctly identifies the intermolecular force represented by B and compares its strength relative to the intermolecular force represented by C?
B represents ion-dipole forces, which are weaker than the force represented by C.
B represents dipole-dipole forces, which are weaker than the force represented by C.
B represents ion-dipole forces, which are stronger than the force represented by C.
B represents dipole-dipole forces, which are stronger than the force represented by C.
A concept map for four types of intermolecular forces and a certain type of bond is shown.
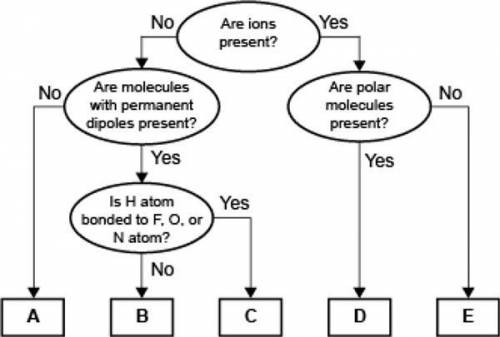

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1
You know the right answer?
Which of the following correctly identifies the intermolecular force represented by B and compares i...
Questions in other subjects:


Physics, 22.03.2020 03:32

Advanced Placement (AP), 22.03.2020 03:32

Mathematics, 22.03.2020 03:32

Mathematics, 22.03.2020 03:32



Mathematics, 22.03.2020 03:32


Biology, 22.03.2020 03:32



