
Chemistry, 18.12.2020 18:00 esme06quirino
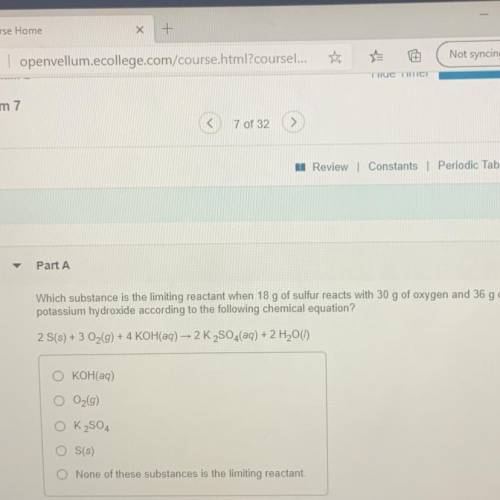
Which substance is the limiting reactant when 18 g of sulfur reacts with 30 g of oxygen and 36 g of
potassium hydroxide according to the following chemical equation?
2 S(s) + 3 O2(g) + 4 KOH(aq) - 2 K2SO4(aq) + 2 H2O(1)
O KOH(aq)
O2(g)
O K2SO4
O S(s)
O None of these substances is the limiting reactant.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 23.06.2019 04:31, woodfordmaliky
One student said that the investigation was not valid (a fair test). write a plan for the investigation that includes improvements to the method and apparatus
Answers: 1
You know the right answer?
Which substance is the limiting reactant when 18 g of sulfur reacts with 30 g of oxygen and 36 g of...
Questions in other subjects:






Mathematics, 28.09.2021 08:00



Chemistry, 28.09.2021 08:00



