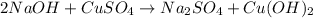Chemistry, 18.12.2020 17:30 Wolfzbayne
You react 14.5 grams of sodium hydroxide with 14.5 grams of copper II sulfate. At the end of the lab you find that you recovered 7.99 grams of precipitate. When you write the balanced equation for you reaction, what is the coefficient in front of sodium hydroxide?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, alvaradolm6853
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1

Chemistry, 22.06.2019 21:20, carlydays4403
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 00:00, glocurlsprinces
(04.05 hc) analyze the given diagram of the carbon cycle below. part 1: which compound does c represent? part 2: name a process that could release this compound into the air. part 3: explain how the elements that form it are conserved during the carbon cycle. use complete sentences to explain your answer. justify how this compound was created from a recycling of carbon in the carbon cycle. use complete sentences to explain your answer.
Answers: 3

Chemistry, 23.06.2019 00:00, jasmin5285
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
You react 14.5 grams of sodium hydroxide with 14.5 grams of copper II sulfate. At the end of the lab...
Questions in other subjects:


History, 13.08.2021 15:50



History, 13.08.2021 15:50


Mathematics, 13.08.2021 15:50

Mathematics, 13.08.2021 15:50

Arts, 13.08.2021 15:50

World Languages, 13.08.2021 16:00