GIVING BRAINLISTED
PLEASE ONLY ANSWER IF YOU KNOW IT
REALLY DONT BE THAT GUY
Ques...

Chemistry, 18.12.2020 04:00 sabahtramirez01
GIVING BRAINLISTED
PLEASE ONLY ANSWER IF YOU KNOW IT
REALLY DONT BE THAT GUY
Question :
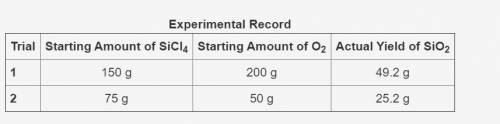
In the following reaction, oxygen is the excess reactant.
SiCl4 + O2 → SiO2 + Cl2
The table shows an experimental record for the above reaction.
-> THE TABLE IS IN THE IMAGE <-
Calculate the percentage yield for SiO2 for Trial 1. Also, determine the leftover reactant for the trial. Show your work.
Based on the percentage yield in Trial 2, explain what ratio of reactants is more efficient for the given reaction.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, savag3jitt
In all living cells dna controls cellular activities by
Answers: 1

Chemistry, 22.06.2019 06:30, cadenhuggins2
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 14:30, Kiaraboyd9366
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
Questions in other subjects:






History, 26.07.2019 20:30

Biology, 26.07.2019 20:30





