
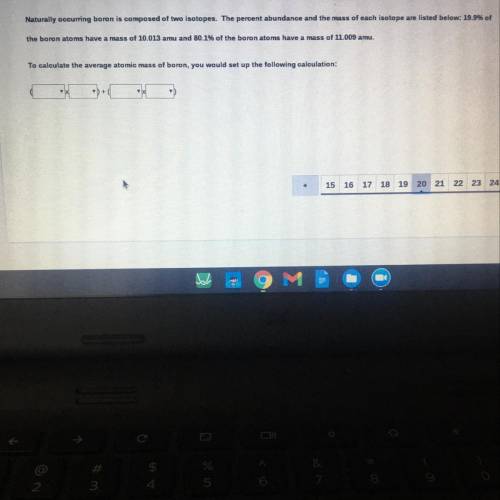
Naturally occurring boron is composed of two isotopes. The percent abundance and the mass of each isotope are listed below: 19.99
the boron atoms have a mass of 10.013 amu and 80.1% of the boron atoms have a mass of 11.009 amu.
To calculate the average atomic mass of boron, you would set up the following calculation:
+

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, daniel1480
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 21:00, estherdinhllama
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 23.06.2019 01:00, Johnson926
Which elements are found in glucose, the product of photosynthesis? a. carbon, hydrogen, and oxygen b. carbon and hydrogen c. carbon, nitrogen, and oxygen d. hydrogen, nitrogen, and carbon
Answers: 2

You know the right answer?
Naturally occurring boron is composed of two isotopes. The percent abundance and the mass of each is...
Questions in other subjects:

Mathematics, 04.12.2020 09:20


Chemistry, 04.12.2020 09:20



World Languages, 04.12.2020 09:20

Health, 04.12.2020 09:20


Mathematics, 04.12.2020 09:20




