Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, KnMcdonaldk93906
Which substances have the lowest melting points: ionic covalent, or metallic
Answers: 1

Chemistry, 22.06.2019 00:00, lukeakalucas
Alarge marble is dropped in a graduated cylinder with 35ml of water in it. the water level increases to 49ml. what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 10:10, veronica022
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
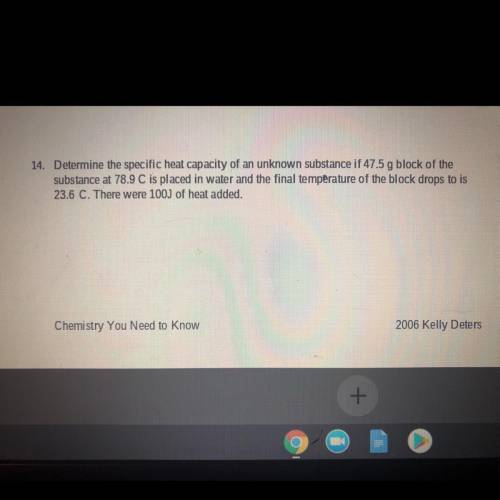
Determine the specific heat capacity of an unknown substance if 47.5 g block of the substance at 78....
Questions in other subjects:


English, 01.02.2021 18:30

Physics, 01.02.2021 18:40

Social Studies, 01.02.2021 18:40


Mathematics, 01.02.2021 18:40

Mathematics, 01.02.2021 18:40

English, 01.02.2021 18:40


Mathematics, 01.02.2021 18:40