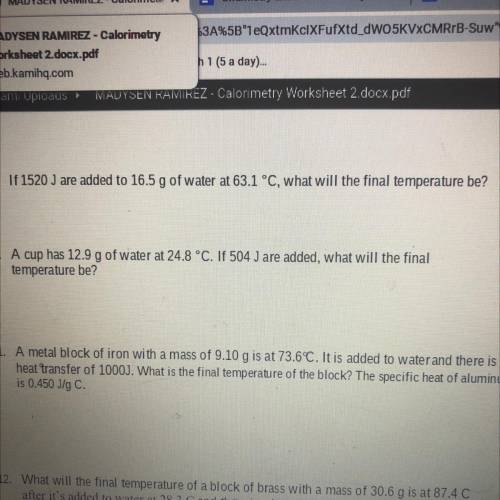
If 1520 J are added to 16.5 g of water at 63.1 c what will the final temperature be
...

Chemistry, 17.12.2020 07:40 dsaefong00
If 1520 J are added to 16.5 g of water at 63.1 c what will the final temperature be

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, sierravick123owr441
Asap! ! who wants to me with my assignment : ) (especially with how i’m gonna draw out the example) you have explored some interesting, informative, and amusing examples of models. now it's time to get creative and make your own model. here is the requirement checklist for your model: ✔ model types can include drawings, diagrams, physical models, virtual simulations, or videos. ✔ models must be created by you, not something selected from an online or outside source. ✔ submit a presentation, picture, video, or screenshot of your model. ✔ submit a one-paragraph summary describing the topic you chose, your model, what it represents, how you made it, and the specific science involved. it is important that you are using science terminology and are accurate. now that you know how to create and submit your model, you will need to choose a topic for your model. choose one of the three topics listed below. select each topic for an overview. -conservation of mass -atomic theory -thermal energy
Answers: 2

Chemistry, 22.06.2019 03:30, ilizzy1224
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 11:30, samantha9430
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 22.06.2019 12:50, khorasanpublic
The number at the end of an isotope’s name is the number.
Answers: 1
You know the right answer?
Questions in other subjects:


Mathematics, 03.02.2020 00:49

Biology, 03.02.2020 00:49


History, 03.02.2020 00:49



Mathematics, 03.02.2020 00:49




