Write the net ionic equation for the reaction between MnO4- ions and H2O2 in acid solution.
...

Chemistry, 16.12.2020 23:00 JoshuaXYP9978
Write the net ionic equation for the reaction between MnO4- ions and H2O2 in acid solution.
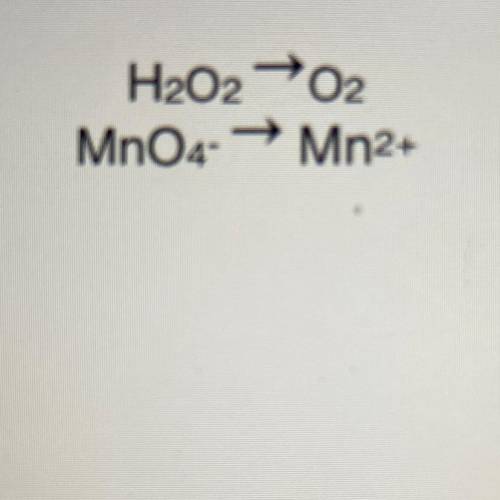

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, mil3ndy
Activity two: just lemons, inc. production here's a one-batch sample of just lemons lemonade production. determine the percent yield and amount of leftover ingredients for lemonade production and place your answers in the data chart. hint: complete stoichiometry calculations for each ingredient to determine the theoretical yield. complete a limiting reactant-to-excess reactant calculation for both excess ingredients. water sugar lemon juice lemonade percent yield leftover ingredients 946.36 g 196.86 g 193.37 g 2050.25 g 89% just lemons lemonade recipe equation: 2 water + sugar + lemon juice = 4 lemonade mole conversion factors: 1 mole of water = 1 cup = 236.59 g 1 mole of sugar = 1 cup = 225 g 1 mole of lemon juice = 1 cup = 257.83 g 1 mole of lemonade = 1 cup = 719.42 g show your calculations below. analysis questions 1. based on taste observations only, which ingredients were in excess in the lemonade samples in activity one? in activity one the excess substances for each sample were the water and sugar. 2. based on the data in activity two, which excess ingredients are affecting the taste of the lemonade in the sample batch? 3. what can just lemons, inc. do during production to reduce the amount of excess ingredients and improve the taste of their lemonade? 4. try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. show your stoichiometric calculations below. 5. during factory inspection, just lemons, inc. discovered that a water valve to the lemonade mixing station was not functioning. once they repair it, more water will enter the mixing station. from what you know about the limiting and excess ingredients for current lemonade production, what advice would you give engineers about the upcoming increase in water?
Answers: 1

Chemistry, 21.06.2019 16:10, amuijakobp78deg
Agas mixture with a total pressure of 745 mmhg contains each of the following gases at the indicated partial pressures: co2, 245 mmhg ; ar, 119 mmhg ; and o2, 163 mmhg . the mixture also contains helium gas. part a what is the partial pressure of the helium gas? phe p h e = nothing mmhg request answer part b what mass of helium gas is present in a 10.2-l sample of this mixture at 283 k ? m m = nothing g request answer
Answers: 1

Chemistry, 21.06.2019 23:00, ayoismeisalex
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2
You know the right answer?
Questions in other subjects:

Mathematics, 02.08.2019 18:00

Mathematics, 02.08.2019 18:00

Health, 02.08.2019 18:00

History, 02.08.2019 18:00


English, 02.08.2019 18:00




History, 02.08.2019 18:00



