
Chemistry, 16.12.2020 14:00 ashvinmsingh
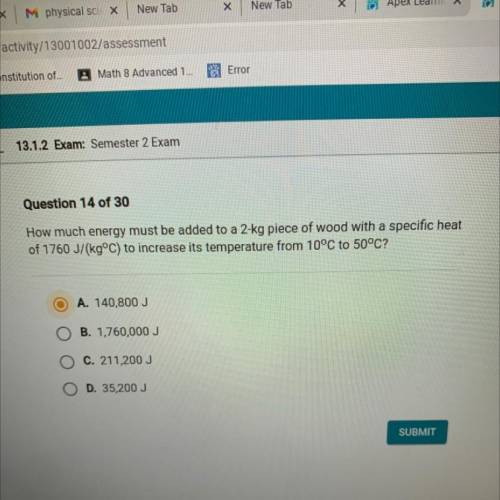
How much energy must be added to a 2-kg piece of wood with a specific heat
of 1760 J/(kg°C) to increase its temperature from 10°C to 50°C?
A. 140,800 J
B. 1,760,000 J
C. 211,200 J
D. 35,200 J

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, breannaking9734
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2


Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

You know the right answer?
How much energy must be added to a 2-kg piece of wood with a specific heat
of 1760 J/(kg°C) to incr...
Questions in other subjects:







Biology, 14.01.2020 21:31



Physics, 14.01.2020 21:31



