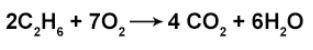How many moles of H2O are produced when 3.25 moles of O2 react in the above equation?
a
12.92...


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, dgadam7495
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 03:10, josephpezza18
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1


Chemistry, 22.06.2019 04:30, logan12345677885675
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2
You know the right answer?
Questions in other subjects:


Computers and Technology, 08.10.2019 18:30

English, 08.10.2019 18:30





Biology, 08.10.2019 18:30


Mathematics, 08.10.2019 18:30