Lab: Acids and Bases
Virtual Lab
Active
Step 3: Create a Model Solution of pH =6 with M...

Chemistry, 16.12.2020 02:50 Wanna14ever
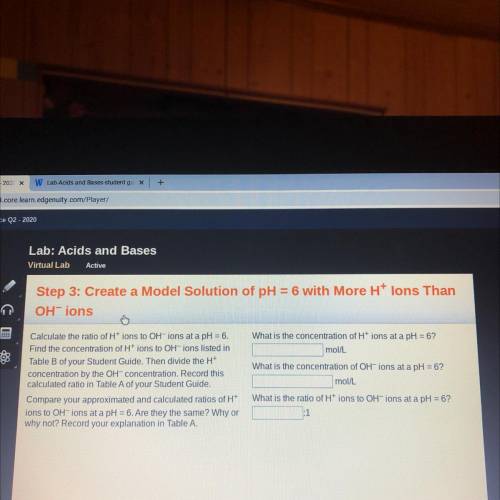
Lab: Acids and Bases
Virtual Lab
Active
Step 3: Create a Model Solution of pH =6 with More Ht lons Than
OHT ions
Calculate the ratio of Ht ions to OH ions at a pH=6.
Find the concentration of Ht ions to OHT ions listed in
Table B of your Student Guide. Then divide the Ht
concentration by the OHT concentration. Record this
calculated ratio in Table A of your Student Guide.
Compare your approximated and calculated ratios of H
ions to OH ions at a pH =6.Are they the same? Why or
why not? Record your explanation in Table A.
What is the concentration of Ht ions at a pH= 6?
mol/l.
What is the concentration of OH ions at a pH =6?
mollL
What is the ratio of Ht ions to OH
ions at a pH6?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1

Chemistry, 22.06.2019 14:30, Priskittles
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
Questions in other subjects:




History, 13.04.2020 22:32

History, 13.04.2020 22:32

History, 13.04.2020 22:32

English, 13.04.2020 22:32



English, 13.04.2020 22:32



