
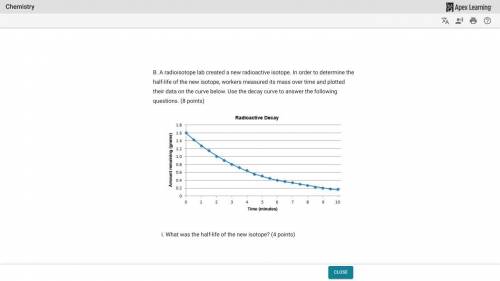
A radioisotope lab created a new radioactive isotope. In order to determine the half-life of the new isotope, workers measured its mass over time and plotted their data on the curve below.
What was the half-life of the new isotope?
How much of the sample had decayed after 9 minutes?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

You know the right answer?
A radioisotope lab created a new radioactive isotope. In order to determine the half-life of the new...
Questions in other subjects:




Mathematics, 26.02.2021 03:00

English, 26.02.2021 03:00

Mathematics, 26.02.2021 03:00







