
Chemistry, 15.12.2020 21:50 giulianna41
Please Help!
Your car has 1.95 gallons of gasoline (octane, d = 0.6916 g/mL), which reacts with oxygen
according to the balanced reaction below. Your car uses the energy produced by this reaction
at a rate of 115 kJ per second while traveling at a speed of 65 miles per hour. Calculate the
distance (in miles) the car can travel using this amount of octane.
2 C8H18(g) + 25 O2(g) > 16 CO2(g) + 18 H2O(g) ΔHrxn = -10,900 kJ

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, EMQPWE
In a spacecraft, the following reaction occurs: co2(g) + 2lioh(s) -> lico3(s) + h2o(i) (i attached picture of equation) how many liters of carbon dioxide will 4 moles of lithium hydroxide (lioh) absorb? (one mole of any gads occupies 22.4 l under certain conditions of temperature and pressure. assume those conditions for this equation.) 45l 6.0l 3.0l 34l
Answers: 1

Chemistry, 22.06.2019 06:30, reecedstceklein
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 09:50, revlonknox6
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2

Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3
You know the right answer?
Please Help!
Your car has 1.95 gallons of gasoline (octane, d = 0.6916 g/mL), which reacts with oxy...
Questions in other subjects:


Mathematics, 09.09.2020 14:01

Mathematics, 09.09.2020 14:01

Physics, 09.09.2020 14:01

History, 09.09.2020 14:01


Mathematics, 09.09.2020 14:01

Mathematics, 09.09.2020 14:01

English, 09.09.2020 14:01

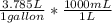 = 7380.75 mL
= 7380.75 mL

