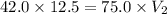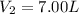Chemistry, 15.12.2020 21:20 Awesomedude1157
10.0 grams of a gas occupies 12.5 liters at a pressure of 42.0 mm Hg. What is the volume when the pressure has increased to 75.0 mm Hg? 6.72 L 7.00 L 22.3 L 0.143 L

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 15:20, KhloodAhmed
Plzzz ? which stores information in discrete steps? a magnet and coil of wire compact discs plastic records amplified speakers
Answers: 2

Chemistry, 23.06.2019 15:50, tatiaa2155
Many radioactive atoms that have large masses undergo radioactive decay by releasing a particle that is identical to a helium-4 nucleus. what changes in the original atom are expected as a result of this natural phenomenon? the atomic number and the mass number will decrease. the atomic number and the mass number will increase. the atomic number will increase, and the mass number will decrease. the atomic number will decrease, and the mass number will increase.
Answers: 2

Chemistry, 23.06.2019 17:50, keshewar2671
The only bonds in a formula unit of caf2 are nonpolar covalent polar covalent ionic metallic
Answers: 1
You know the right answer?
10.0 grams of a gas occupies 12.5 liters at a pressure of 42.0 mm Hg. What is the volume when the pr...
Questions in other subjects:

Mathematics, 26.05.2021 16:30


Health, 26.05.2021 16:30


Mathematics, 26.05.2021 16:30


Chemistry, 26.05.2021 16:30



Mathematics, 26.05.2021 16:30

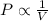 (At constant temperature and number of moles)
(At constant temperature and number of moles)
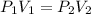
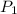 = initial pressure of gas = 42.0 mm Hg
= initial pressure of gas = 42.0 mm Hg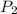 = final pressure of gas = 75.0 mm Hg
= final pressure of gas = 75.0 mm Hg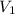 = initial volume of gas = 12.5 L
= initial volume of gas = 12.5 L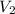 = final volume of gas = ?
= final volume of gas = ?