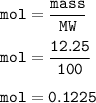Chemistry, 15.12.2020 14:00 royaltyy6533
A scientist completes a thermal decomposition reaction. 12.25g of calcium carbonate, CaCO3, is heated
and forms calcium oxide, Cao, and carbon dioxide, CO2.
a. Write a balanced chemical equation to demonstrate this reaction. Include state symbols.
b. Calculate the number of moles of calcium carbonate that is thermally decomposed in this
reaction.
Ok

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 23.06.2019 00:30, joshsmith2022
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 03:30, HalpMahOnMahH0meW0rk
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1

You know the right answer?
A scientist completes a thermal decomposition reaction. 12.25g of calcium carbonate, CaCO3, is heate...
Questions in other subjects:

Mathematics, 22.09.2020 04:01



Social Studies, 22.09.2020 04:01





Mathematics, 22.09.2020 04:01

Spanish, 22.09.2020 04:01