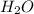Chemistry, 15.12.2020 04:00 danielboek
How many molecules of oxygen are produced when a sample of 38.9 g of water is decomposed by electricity?
A) 6.5 x 10^23
B) 1.3 x 10^24
C) 2.6 x 10^24
D) 2.3 x 10^25

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:20, anggar20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 00:30, jamesnaquan132
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 01:20, sarinaneedshelp01
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 01:30, kayleg907436
Arollercoaster car at the top of a hill has potential energy kinetic energy chemical energy light energy
Answers: 1
You know the right answer?
How many molecules of oxygen are produced when a sample of 38.9 g of water is decomposed by electric...
Questions in other subjects:

Mathematics, 21.01.2021 21:40



Mathematics, 21.01.2021 21:40

Mathematics, 21.01.2021 21:40

Mathematics, 21.01.2021 21:40

Biology, 21.01.2021 21:40




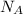 = Avogadro's number =
= Avogadro's number =