
Chemistry, 14.12.2020 20:30 clietmaster112
Gas law A gas has a volume of 350 mL at 4.5 ATM. If the volume changes to 400 mL, what is the new pressure? The temperature in the number of moles stay the same.
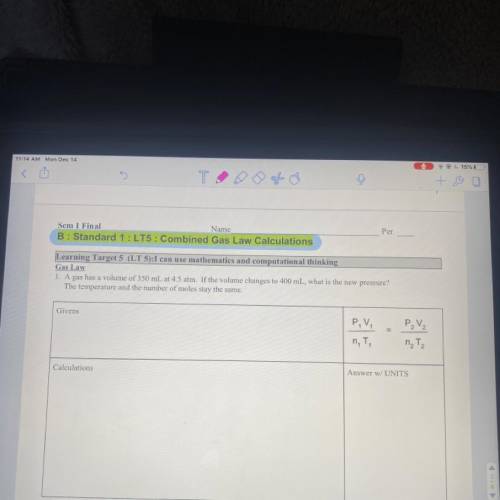

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, TamB01
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 06:00, girly37
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3


Chemistry, 22.06.2019 19:20, halledoll2002
Anyone who's in connections academy chemistry b have the factors that affect the rate of a reaction portfolio already done?
Answers: 3
You know the right answer?
Gas law
A gas has a volume of 350 mL at 4.5 ATM. If the volume changes to 400 mL, what is the new p...
Questions in other subjects:

Law, 27.07.2021 01:00


English, 27.07.2021 01:00

Engineering, 27.07.2021 01:00


Mathematics, 27.07.2021 01:00



Biology, 27.07.2021 01:00

Mathematics, 27.07.2021 01:00



