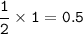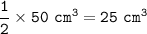4 Nitrogen monoxide reacts with oxygen like this:
2NO(gas) + O2(gas) -> 2NO2 (gas)
a. How m...

4 Nitrogen monoxide reacts with oxygen like this:
2NO(gas) + O2(gas) -> 2NO2 (gas)
a. How many moles of oxygen molecules react
with I mole of nitrogen monoxide molecules?
b. What volume of oxygen will react with
50 cm of nitrogen monoxide?
c. Using the volumes in b, what is:
i. the total volume of the two reactants?
ii. the volume of nitrogen dioxide formed?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, carlybeavers50
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 05:30, jzjajsbdb8035
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
Questions in other subjects:


History, 02.07.2019 16:50







Spanish, 02.07.2019 16:50

English, 02.07.2019 16:50