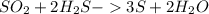Chemistry, 14.12.2020 14:00 AdrianBrewer8168
How many moles of sulfur dioxide (SO2) are required to produce 5.0 moles of sulfur (S) according to the following balanced equation? SO2 + 2H2S - 3S + 2H2O

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, pressure772
Which is a character of nuclear fusion but not nuclear fission
Answers: 3



Chemistry, 22.06.2019 08:00, anglacx5465
Why are pipes bursting in the in extremely cold weather?
Answers: 2
You know the right answer?
How many moles of sulfur dioxide (SO2) are required to produce 5.0 moles of sulfur (S) according to...
Questions in other subjects:



Chemistry, 23.10.2019 08:00

Mathematics, 23.10.2019 08:00



Mathematics, 23.10.2019 08:00

Mathematics, 23.10.2019 08:00