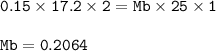A student used a pipette to add 25.0 cm of KOH of unknown concentration to
a conical flask.
Th...

Chemistry, 13.12.2020 23:20 bekahmc1p6k6vj
A student used a pipette to add 25.0 cm of KOH of unknown concentration to
a conical flask.
The student carried out a titration experiment to find the volume of 0.150 mol/dmº
H2SO4 needed to neutralise the KOH.
The student found that, on average, 17.20 cm of the H2SO4 solution was
required for neutralisation.
Calculate the concentration of the KOH solution.

Answers: 1


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 19:00, QuestionsAnsweredNow
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 26.06.2019 15:30

Biology, 26.06.2019 15:30



World Languages, 26.06.2019 15:30

Mathematics, 26.06.2019 15:30


History, 26.06.2019 15:30

Physics, 26.06.2019 15:30