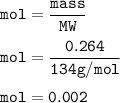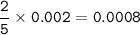Chemistry, 13.12.2020 08:20 barbie8775
0.2640 g of sodium oxalate is dissolved in a flask and requires 30.74 mL of potassium
permanganate (from a buret) to titrate it and cause it to turn pink (the end point).
The equation for this reaction is:
5Na2C2O4(aq) + 2KMnO4(aq) + 8H2SO4(aq) ---> 2MnSO4(aq) + K2SO4(aq) + 5Na2SO4(aq) +
10CO2(g) + 8H2O(1)
(A) How many moles of potassium permanganate have been titrated into the flask to reach the end point

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Aidanjsauer
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 11:00, snowprincess99447
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 23.06.2019 00:30, natishtaylor1p8dirz
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3

Chemistry, 23.06.2019 09:30, kleathers97
Hey, could someone me answer this? much appreciated!
Answers: 1
You know the right answer?
0.2640 g of sodium oxalate is dissolved in a flask and requires 30.74 mL of potassium
permanganate...
Questions in other subjects:



Mathematics, 10.01.2020 23:31

Chemistry, 10.01.2020 23:31


Business, 10.01.2020 23:31


Mathematics, 10.01.2020 23:31