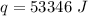Chemistry, 13.12.2020 07:00 nautiannaharper59901
How much heat is required to take a 150 g sample of water from 10.0 ℃ to 95.0 ℃? cs, water = 4.184 J/g*℃

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alexmarche4675
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2


Chemistry, 22.06.2019 09:00, SilverTheAmarok
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2
You know the right answer?
How much heat is required to take a 150 g sample of water from 10.0 ℃ to 95.0 ℃? cs, water = 4.184 J...
Questions in other subjects:


Mathematics, 19.08.2021 03:30

History, 19.08.2021 03:30

History, 19.08.2021 03:30






Chemistry, 19.08.2021 03:30