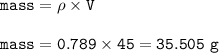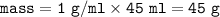Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 22.06.2019 17:00, abbygailgo674
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
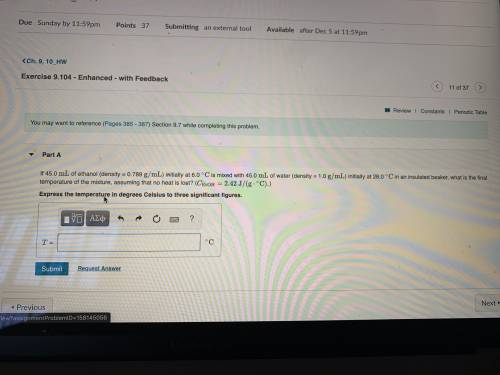
If 45.0 mL of ethanol (density =0.789g/mol) initially at 6.0°C mix with 45.0 mL of water (density =1...
Questions in other subjects:

History, 03.12.2020 22:00

Mathematics, 03.12.2020 22:00

English, 03.12.2020 22:10


Mathematics, 03.12.2020 22:10




Mathematics, 03.12.2020 22:10

Mathematics, 03.12.2020 22:10