Chemistry, 12.12.2020 17:10 Angelanova69134
What is the molality of a D-glucose solution prepared by dissolving 36.0 g of D-glucose, C6H12O6, in 125.9 g of water

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 23:00, hailey5campbelp7d1c0
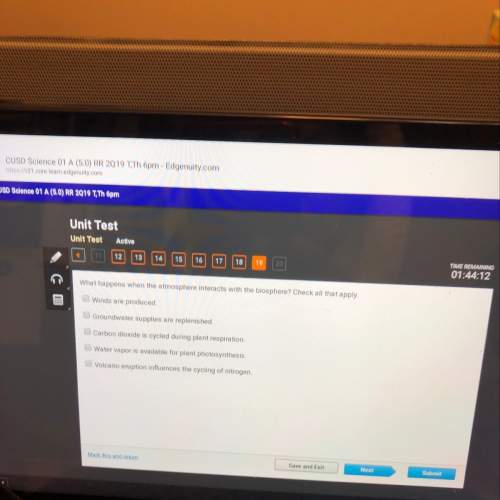
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2

Chemistry, 23.06.2019 00:30, evelynalper08
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2
You know the right answer?
What is the molality of a D-glucose solution prepared by dissolving 36.0 g of D-glucose, C6H12O6, in...
Questions in other subjects:

Social Studies, 24.11.2021 18:20


English, 24.11.2021 18:20


Biology, 24.11.2021 18:20

Social Studies, 24.11.2021 18:20




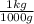 = 0.1259 kg H₂O
= 0.1259 kg H₂O