
Chemistry, 12.12.2020 16:30 simrankaurdhatt
PLZ HELP
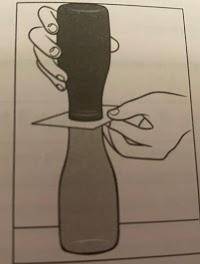
A group of students simulates colliding air masses. Using an index card for separation, a jar of warm tap water with red food coloring is placed over a jar of cold water with yellow food coloring. The index card that separates the two bodies of water is removed. Students observe how the two fronts travel in relation to one another.
A.) Air masses are not distinguisable by color.
B.) Air masses are neither hot nor cold in temperature
C.) Air masses do not collide in real life
D.) Air masses cannot be modeled

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 23.06.2019 00:50, lakhanir2013
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

Chemistry, 23.06.2019 03:00, jaidencoolman2866
Determine type of reaction & predict the product c3h12+o2 =
Answers: 1

Chemistry, 23.06.2019 03:00, yoongislaugh
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
PLZ HELP
A group of students simulates colliding air masses. Using an index card for separation, a...
Questions in other subjects:

Physics, 09.03.2022 17:50




Mathematics, 09.03.2022 17:50


Mathematics, 09.03.2022 17:50

English, 09.03.2022 17:50


Engineering, 09.03.2022 17:50



