
Chemistry, 21.09.2019 01:40 dbenjamintheflash5
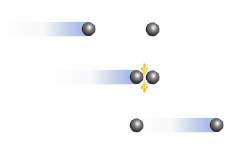
Consider the diagram. which postulate of the kinetic-molecular theory best describes the event in the diagram?
a. most of the volume of a gas is empty space.
b. all collisions between particles are perfectly elastic.
c. there is no force of attraction or repulsion between gas particles.
d. the average kinetic energy of particles depends only on temperature.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1


Chemistry, 22.06.2019 14:30, amylumey2005
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
Consider the diagram. which postulate of the kinetic-molecular theory best describes the event in th...
Questions in other subjects:


Mathematics, 17.04.2020 02:48



Mathematics, 17.04.2020 02:48

Mathematics, 17.04.2020 02:48

History, 17.04.2020 02:48



Mathematics, 17.04.2020 02:48



