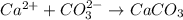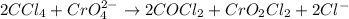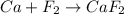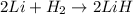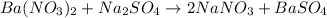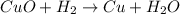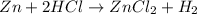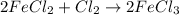Chemistry, 11.10.2019 04:30 julianbeaver76
Classify these reactions according to the type discussed in the chapter.
b) ca2+ + co32- -> caco3
d) 2ccl4 + cro42- -> 2cocl2+ cro2cl2+2cl-
e) ca+ f2-> caf2
f) 2li+h2-> 2lih
g) ba(no3)2+na2so4-> 2nano3+baso4
h) cuo+h2-> cu+h20
i) zn+2hcl-> zncl2+h2
j) 2fecl2+cl2-> 2fecl3

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:00, itasykamila
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 00:30, mathwiznot45
Element j is 1s 2s 2p 3s . (i) how many unpaired electrons does j have? (ii) is j a good oxidizing agent or a reducing agent? (iii) state reason for the answer.
Answers: 1


Chemistry, 23.06.2019 02:30, ijustneedhelp29
Which of the following statements are incorrect?
Answers: 3
You know the right answer?
Classify these reactions according to the type discussed in the chapter.
b) ca2+ + co32...
b) ca2+ + co32...
Questions in other subjects:


Mathematics, 09.07.2019 23:10


English, 09.07.2019 23:10

Mathematics, 09.07.2019 23:10

Mathematics, 09.07.2019 23:10