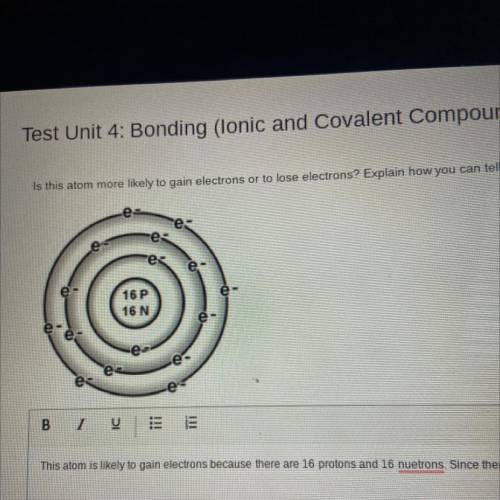
Is this atom more likely to gain electrons or to lose electrons? Explain how you can tell
e
e...

Chemistry, 11.12.2020 01:50 eazywalters
Is this atom more likely to gain electrons or to lose electrons? Explain how you can tell
e
e-
e е
es
e е
16P
16 N
e es
Please help

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, chrisxxxrv24
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2


Chemistry, 23.06.2019 09:00, cathyfrawley
Chortling is used to clean water. another possible atom that would also work is a. sodium b. sulfur c. bromine
Answers: 1
You know the right answer?
Questions in other subjects:




English, 11.12.2019 17:31

Mathematics, 11.12.2019 17:31


Health, 11.12.2019 17:31


Social Studies, 11.12.2019 17:31



