
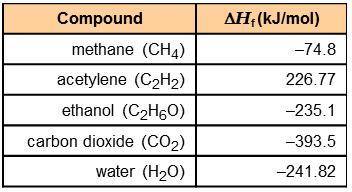
Using the information in the table to the right, calculate the enthalpy of combustion of each of the following substances:
acetylene:
ethanol:
The combustion of 0.25 mol of an unknown organic compound results in the release of 320 kJ of energy. Which of the compounds in the table could be the unknown compound?
ANSWERS:
1.
acetylene: -1,256 kJ/mol
ethanol: -1,277 kJ/mol
2.
ethanol
I already know the answers (they're right there ^) i just need to know HOW you find the enthalpy combustion of acetylene and ethanol.

Answers: 3


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 17:40, adantrujillo1234
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 23:30, johnnysteeler9934
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
Using the information in the table to the right, calculate the enthalpy of combustion of each of the...
Questions in other subjects:





Mathematics, 12.08.2020 06:01







