
Chemistry, 10.12.2020 23:40 fatherbamboo
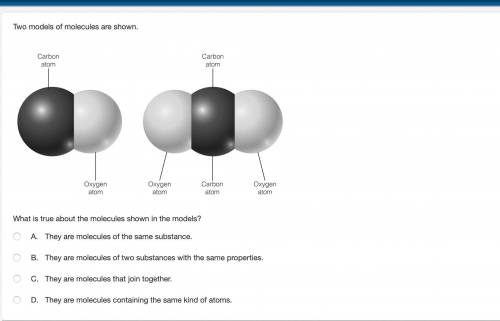
Two models of molecules are shown. What is true about the molecules shown in the models? The one on the left has 1 carbon atom and 1 oxygen atom and the one on the right has 2 oxygen atoms and 1 carbon atom?
A. They are molecules of the same substance.
B. They are molecules of two substances with the same properties.
C. They are molecules that join together.
D. They are molecules containing the same kind of atoms.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 16:00, anaalashay
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

Chemistry, 22.06.2019 17:10, sophiaa23
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1
You know the right answer?
Two models of molecules are shown. What is true about the molecules shown in the models? The one on...
Questions in other subjects:

Mathematics, 29.01.2021 16:30












