

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, gonzaleze18
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 13:00, monkeyrose1999
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3
You know the right answer?
Calculate the maximum volume in mL of 0.20 M HCl that a tablet containing 338 mg Al(OH)3 and 489 mg...
Questions in other subjects:

Social Studies, 28.07.2019 13:50

History, 28.07.2019 13:50







Business, 28.07.2019 13:50

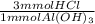 = 13 mmol HCl
= 13 mmol HCl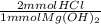 = 16.77 mmol HCl
= 16.77 mmol HCl

