
Ammonia (A) diffuses through a stagnant layer of air (B), 1cm thick, at 25 ºC and 1 atm total pressure. The partial pressures of ammonia on the two sides of the air layer are: PA0=0.9 atm and PAl=0.1 atm respectively. Air is none diffusing. Calculate the molar flux of ammonia. DAB= 0.214 cm2 /s

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:10, lindseyklewis1p56uvi
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 22.06.2019 11:50, robert7248
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 12:30, AlexRavenwood127
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 17:30, Naysa150724
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
Ammonia (A) diffuses through a stagnant layer of air (B), 1cm thick, at 25 ºC and 1 atm total pressu...
Questions in other subjects:


English, 18.09.2021 14:00


Mathematics, 18.09.2021 14:00


Geography, 18.09.2021 14:00


History, 18.09.2021 14:00


Business, 18.09.2021 14:00

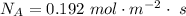
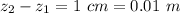
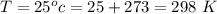



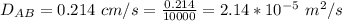
![N_A = \frac{D_{AB} * P_T }{RT(z_2 -z_1)} * ln [\frac{P_T - P_{Al}}{P_T - P_{AO}} ]](/tpl/images/0969/5009/086d8.png)
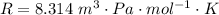
![N_A = \frac{2.14 *10^{-5} * 1.01325*10^{5} }{8.314 *298 (0.01)} * ln [\frac{1 - 0.1}{1 - 0.9} ]](/tpl/images/0969/5009/c7bba.png)


