

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 23.06.2019 01:30, rubyr9975
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1

Chemistry, 23.06.2019 01:30, heavendl13
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 06:30, morganzahn16
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1
You know the right answer?
If 25.0 mL of 0.100 M Ca(OH)2 is titrated with 0.200 M HNO3, what volume of nitric acid is required...
Questions in other subjects:

History, 12.11.2019 10:31



Mathematics, 12.11.2019 10:31






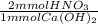 = 5.0 mmol HNO₃
= 5.0 mmol HNO₃

