B. The reaction is spontaeneous

Chemistry, 10.12.2020 07:10 jescanarias22
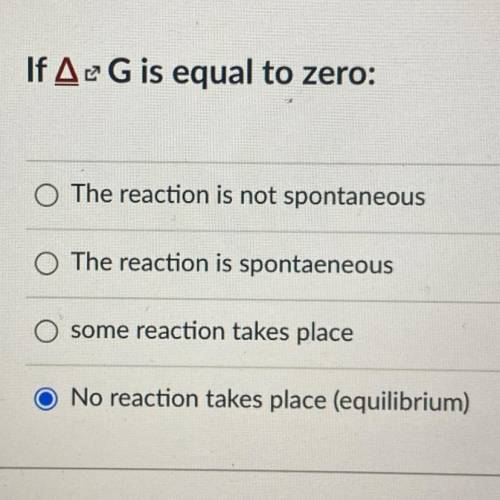
If ΔG is equal to zero:
A. The reaction is not spontaneous
B. The reaction is spontaeneous
C. some reaction takes place
D. No reaction takes place (equilibrium)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, nadinealonzo6121
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2

Chemistry, 22.06.2019 17:00, marsjupiter2554
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1

Chemistry, 23.06.2019 02:50, igraha17
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2
You know the right answer?
If ΔG is equal to zero:
A. The reaction is not spontaneous
B. The reaction is spontaeneous
B. The reaction is spontaeneous
Questions in other subjects:



History, 07.04.2020 07:43


Mathematics, 07.04.2020 07:43

Mathematics, 07.04.2020 07:43






