
Chemistry, 10.12.2020 04:00 lizzyhearts
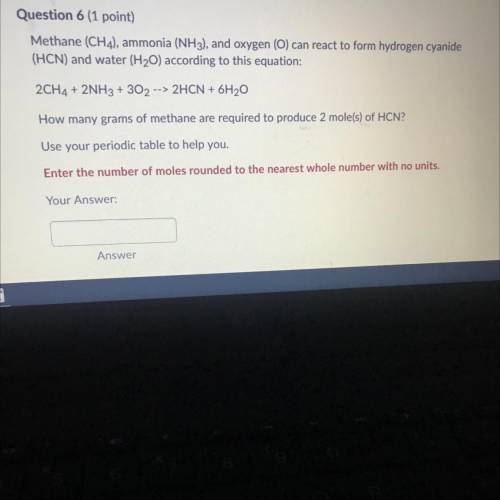
Methane (CH 4 ) , ammonia (NH 3 ) , and oxygen () can reaci to form hydrogen cyanide (HCN) and water (H 2 O) according to this equation 2CH 4 +2NH 3 +3O 2 2HCN+6H 2 O How many grams of methane are required to produce 2 moles ? Use your periodic table to help you. Please show work

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, anthony4034
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3


Chemistry, 22.06.2019 23:30, ninilizovtskt
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
Methane (CH 4 ) , ammonia (NH 3 ) , and oxygen () can reaci to form hydrogen cyanide (HCN) and water...
Questions in other subjects:

Mathematics, 09.03.2021 03:40






Chemistry, 09.03.2021 03:40


Mathematics, 09.03.2021 03:40




