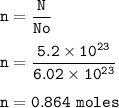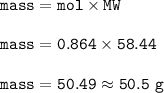4
What is the mass, in grams, of 5.20 x 1023 formula units of NaCl?
A 0.864 grams
11.2...


Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
You know the right answer?
Questions in other subjects:

English, 04.02.2021 22:10



Mathematics, 04.02.2021 22:10



History, 04.02.2021 22:10

Mathematics, 04.02.2021 22:10

English, 04.02.2021 22:10

Mathematics, 04.02.2021 22:10