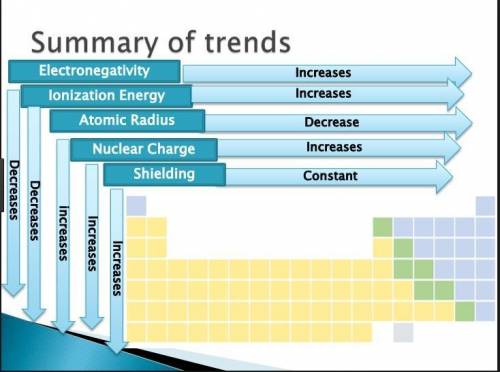
The Periodic Table of the Elements is useful for revealing patterns and trends in the elements. Which statement accurately describes a pattern in the size of atomic radii in the Periodic Table of the Elements?
*Atomic radii decrease from left to right across a period and decrease from top to
bottom in a group.
*Atomic radii increase from left to right across a period and increase from top to
bottom in a group.
*Atomic radii decrease from left to right across a period and increase from top to
bottom in a group.
*Atomic radii increase from left to right across a period and decrease from top to
Wottom in a group.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2



Chemistry, 22.06.2019 19:30, xxaurorabluexx
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
The Periodic Table of the Elements is useful for revealing patterns and trends in the elements. Whic...
Questions in other subjects:


Engineering, 20.01.2020 20:31