
Chemistry, 08.12.2020 16:50 luvberries2276
200.0 mL of 3.85 M HCl is added to 100.0 mL of 4.6 M barium hydroxide. The reaction goes to completion. What is the concentration (in mol/L) of excess H+ or OH- that remains in solution?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1


Chemistry, 22.06.2019 21:30, crystalbyrd79p8imrx
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
200.0 mL of 3.85 M HCl is added to 100.0 mL of 4.6 M barium hydroxide. The reaction goes to completi...
Questions in other subjects:

Arts, 12.11.2020 20:40


Biology, 12.11.2020 20:40

Mathematics, 12.11.2020 20:40


Mathematics, 12.11.2020 20:40




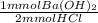 = 101.925 mmol Ba(OH)₂
= 101.925 mmol Ba(OH)₂

