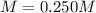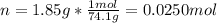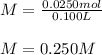Chemistry, 08.12.2020 16:50 gyexisromero10
A saturated solution of calcium hydroxide contains 1.85 g of solute in 100. mL of solution. What is its molarity

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3


Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
A saturated solution of calcium hydroxide contains 1.85 g of solute in 100. mL of solution. What is...
Questions in other subjects:

Mathematics, 19.11.2020 01:30


Mathematics, 19.11.2020 01:30



Spanish, 19.11.2020 01:30


Mathematics, 19.11.2020 01:30

Mathematics, 19.11.2020 01:30