
Chemistry, 08.12.2020 05:50 bbyniah123
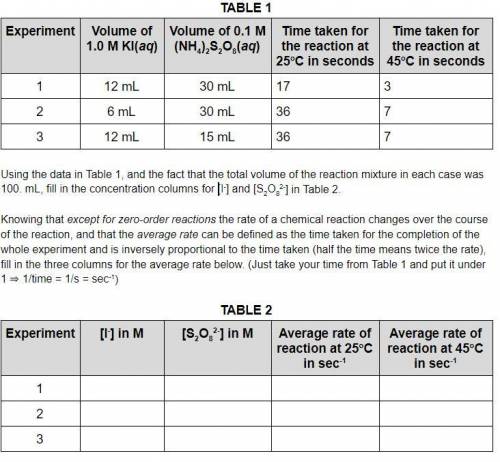
Using the data in Table 1, and the fact that the total volume of the reaction mixture in each case was 100. mL, fill in the concentration columns for [I-] and [S2O82-] in Table 2. Knowing that except for zero-order reactions the rate of a chemical reaction changes over the course of the reaction, and that the average rate can be defined as the time taken for the completion of the whole experiment and is inversely proportional to the time taken (half the time means twice the rate), fill in the three columns for the average rate below. (Just take your time from Table 1 and put it under 1 ⇒ 1/time = 1/s = sec-1)

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 23.06.2019 03:20, elijahmoore841
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
You know the right answer?
Using the data in Table 1, and the fact that the total volume of the reaction mixture in each case w...
Questions in other subjects:

Chemistry, 14.10.2019 19:30



Mathematics, 14.10.2019 19:30


Mathematics, 14.10.2019 19:30







