
Chemistry, 08.12.2020 05:20 rainbowboi
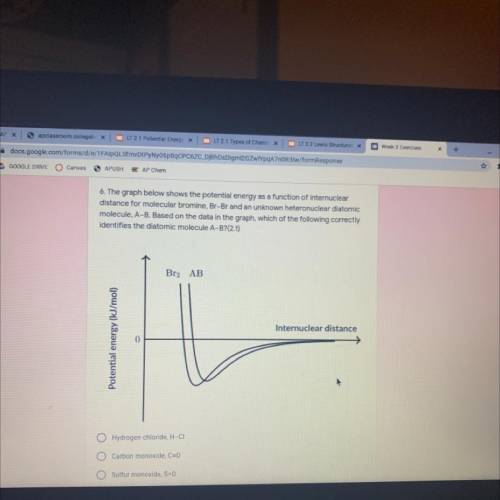
The graph below shows the potential energy as a function of internuclear
distance for molecular bromine, Br-Br and an unknown heteronuclear diatomic
molecule, A-B. Based on the data in the graph, which of the following correctly
identifies the diatomic molecule A-B?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 04:00, breannaasmith1122
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 08:30, audrey1256
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1
You know the right answer?
The graph below shows the potential energy as a function of internuclear
distance for molecular bro...
Questions in other subjects:

Mathematics, 20.01.2021 18:10

Biology, 20.01.2021 18:10

Mathematics, 20.01.2021 18:10


Business, 20.01.2021 18:10




Mathematics, 20.01.2021 18:10




