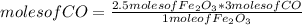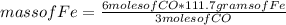Consider the process used to produce iron metal from its ore.
Fe2O3(s) + 3CO(g) --> 2Fe(s) + 3CO2 (g)
How many grams of iron can be produced from 2.5 moles of Fe2O3 and 6.0 moles of CO? Hint: limiting reactant problem
O A. 140 g
B. 335 g
C. 55.858
D. 223 g Fe
E. 279 g Fe

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:10, ChloeLiz7111
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 05:50, mrylenastewart
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 23:00, maddyleighanne
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 01:30, sheldonwaid4278
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
Consider the process used to produce iron metal from its ore.
Fe2O3(s) + 3CO(g) --> 2Fe(s) + 3CO...
Questions in other subjects:

Mathematics, 23.04.2020 03:22

Mathematics, 23.04.2020 03:22

Social Studies, 23.04.2020 03:22



Mathematics, 23.04.2020 03:22



Biology, 23.04.2020 03:22

Mathematics, 23.04.2020 03:22