
Chemistry, 07.12.2020 16:40 twistedhyperboles
The concentration of oxalate ion (C2O42-) in a sample can be determined by titration with a solution of permanganate ion (MnO4-) of known concentration. The net ionic equation for this reaction is 2MnO4- + 5C2O42- + 16H+ --> 2Mn2+ + 8H2O + 10CO2 A 30.00 mL sample of an oxalate solution is found to react completely with 21.93 mL of a 0.1725 M solution of MnO4-. What is the oxalate ion concentration in the sample?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:40, elawnnalewis4855
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 22:40, destineysarah
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 22.06.2019 23:20, svaskeacevilles5477
In medium-sized stars such as the sun, nuclear fusion almost always means the fusing of nuclei to form , but larger stars can produce elements as heavy as
Answers: 2

Chemistry, 23.06.2019 05:30, choatefarmsus
Awhite powder is added to a solution. the images show observations made before the powder is added, just after the powder has been added, and a little while later. (the liquid in the small beaker is phenol red solution.) what evidence shows that a chemical change has taken place?
Answers: 1
You know the right answer?
The concentration of oxalate ion (C2O42-) in a sample can be determined by titration with a solution...
Questions in other subjects:


Geography, 20.09.2019 22:30



Biology, 20.09.2019 22:30

Mathematics, 20.09.2019 22:30




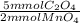 = 9.457 mmol C₂O₄⁻²
= 9.457 mmol C₂O₄⁻² 

